|
|
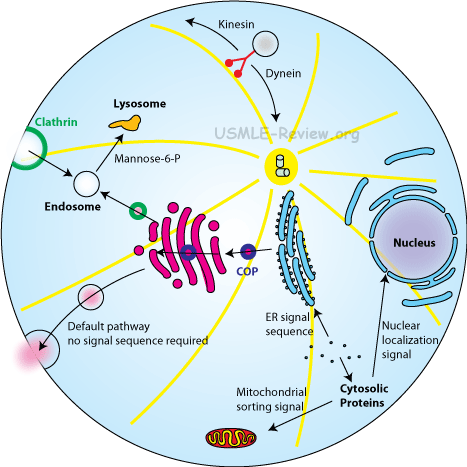
Protein sorting
- Sorting signal (signal sequence) is an amino acid sequence that directs protein to organelle. Cytosol proteins don't have sorting signal.
- Nucleus proteins have nuclear localization signal. Transported in fully folded form through nuclear pores.
- Mitochondrial proteins: some made inside the mitochondria, but most are imported from cytosol. Transported into mitochondria by unfolding and snaking through protein translocator. Chaperones help refold protein once inside. Signal sequence then cleaved off.
- Endoplasmic reticulum
- ER signal sequence targets peptide-ribosome complex to ER.
- Signal recognition particle (SRP) binds signal sequence and guides it to ER.
- SRP receptor on the ER membrane receives the SRP-peptide-ribosome complex.
- Peptide synthesized into the ER lumen via translocator, either as soluble protein or embedded into ER membrane as transmembrane protein.
- Signal sequence cleaved (except for internal signal sequences).
- Transport vesicles then take proteins to Golgi, endosomes, lysosome, plasma membrane, or secretion.
- Lysosomal proteins are tagged with mannose-6-phosphate. I-cell disease = lack of mannose-6-phosphate tagging causes lysosomal proteins to be secreted.
- Resident ER proteins have the KDEL sequence (ER retention signal).
Vesicle trafficking
- Endocytosis: clathrin protein used for phagocytosis, pinocytosis, receptor mediated endocytosis.
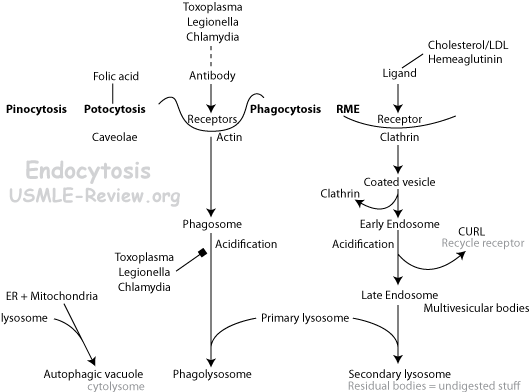
- Pinocytosis: takes in fluids.
- Potocytosis: takes in small molecules like folates via caveolae.
- Phagocytosis: takes in particles. Eg: phagocytes eating bacteria.
- Receptor mediated endocytosis: uses specific receptors in coated pits to form coated vesicles. Eg: update of cholesterol in LDL, or iron in ferritin. Receptors attach to coated pits by adaptin.
- Exocytosis: no protein coat
- Intracellular trafficking: almost all uses COP protein coat.
- Protein coats help recruit and concentrate their cargo, and also deforms the membrane to help budding.
- COP I is used for Golgi → ER
- COP II is used for ER → Golgi.
- Exception to this rule is Golgi → endosome → lysosome, which uses clathrin.
- Cystic fibrosis: mutant chloride channel protein doesn't get transported out of the ER to make it to the plasma membrane.
- Vesicle targeting uses Rab-GTP.
- Vesicles bud off from their target membrane because their coat proteins deform the membrane, and also because dynamin pinches it off by hydrolyzing GTP.
- Membrane fusion uses SNAREs
- v-SNAREs on vesicle membrane.
- t-SNAREs on target membrane.
- v-SNARE and t-SNARE binding confers vesicle-to-target specificity.
- The snares bind and fuse the membranes.
- Transport of vesicles is powered by motor proteins travelling along microtubules.
- Kinesins transport vesicles outward.
- Dyneins transport vesicles inward.
|
|
