|
|
DNA structure: single- and double-stranded DNA, stabilizing forces, supercoiling
- single-stranded DNA: the sequence (primary structure) is the most important structure of ssDNA. Higher level structures for ssDNA are difficult to predict, but may contain elements of base-stacking and looping back on itself to form base-pairs.
- double-stranded DNA:
- Forms a right-handed double helix (B-DNA).
- The two strands are held together by base-pairing: A-T, G-C.
- A-T held together by 2 hydrogen bonds. G-C held together by 3 hydrogen bonds.
- A and G are purines (bigger). T and C are pyrimidines (smaller).
- B-DNA is the right-handed double helix found in our bodies. The A (fat) and Z (left-handed, CpG repeats) forms of DNA are non-native forms.
- Major groove (bigger) and minor groove (smaller) are open spaces in the double helix where drugs and enzymes can bind to.
- Stabilizing forces:
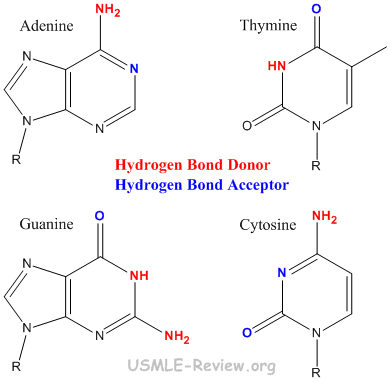
- Base-pairing: A-T, G-C hydrogen bonding holds two DNA strands together.
- Base-stacking: the planar, aromatic bases stack on top of one another via hydrophobic interactions.
- Supercoiling = twisting the double helix so that it coils to relieve the stress.
- Positive supercoiling: twisting the double helix in a right-handed fashion (as if to overwind the double helix).
- Negative supercoiling: twisting the double helix in a left-handed fashion (as if to underwind the double helix).
- Topoisomerase: make or break supercoils.
- Class I topoisomerase: cut only one strand of DNA.
- Class II topoisomerase: cut both strands of DNA.
- DNA Gyrase = bacterial class II topoisomerase.
- Prokaryotic DNA can supercoil because they are circular.
- Eukaryotic DNA can supercoil because they wrap around histones.
analysis of DNA: sequencing, restriction analysis, PCR amplification, hybridization
- DNA Sequencing:
- Primer extension.
- Dideoxy termination.
- Resolution on analytical instrument.
- Restriction analysis: cut double-stranded DNA at certain palindrome sequences with restriction endonuclease.
- PCR amplification = exponential amplification of DNA by repeating the following steps.
- Denaturation: heat to separate dsDNA strands so that primer can anneal.
- Annealing: cool so that primers can anneal to the separated strands.
- Elongation: heat-stable polymerase extends the annealed primers into new complementary DNA strands.
- Hybridization: DNA and RNA with complementary sequences can hybridize to make a DNA-RNA hybrid.
DNA replication, mutation, repair, degradation, and inactivation
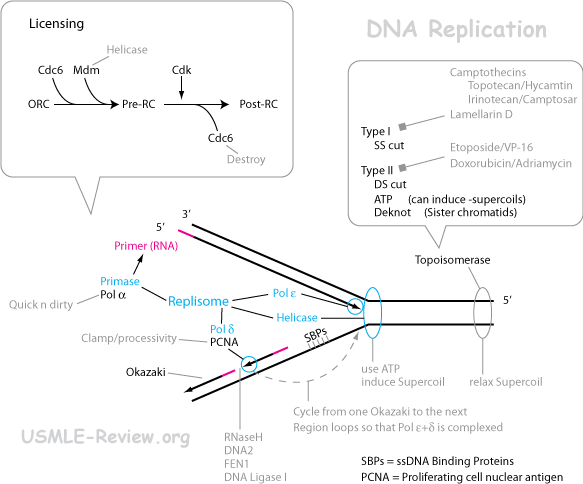
- DNA replication:
- Origin of replication
- DNA synthesis is bidirectional (except in some viruses).
- Prokaryotes have only one origin of replication. Called OriC in E. coli.
- Eukaryotes have multiple origins of replication. Called replicators.
- Origin recognition complex (ORC) binds origin of replication.
- Pre-replication complex (pre-RC) formation via binding of activator (RAP) and licensing factors (RLFs).
- Post-replication complex (post-RC) formation via cyclin-dependent kinases (CDK) phosphorylation and subsequent degradation of pre-RC proteins.
- Initiation: replication can now initiate, but only once, because pre-RC proteins are degraded.
- Unwind double helix
- Helicase unwinds the double helix at the replication fork.
- Single-strand binding proteins (SSB) keep the strands separated and also protect DNA from degradation. In eukaryotes, the SSB is called RPA.
- DNA synthesis
- Primase makes an RNA primer. In eukaryotes, the primase is part of the polymerase. Later, this RNA primer is replaced with DNA. In prokaryotes, this is all done by Pol I, but in eukaryotes, this is done by RNase (RNase H1 and FEN-1) and Pol δ.
- Polymerase makes DNA.
- DNA synthesis always occurs from 5'→3' direction.
- Leading strand makes DNA in an overall 5'→3' direction by continuous 5'→3' DNA synthesis.
- Lagging strand makes DNA in an overall 3'→5' direction by making (Okazaki) fragments that are synthesized 5'→3' direction.
- DNA ligase joins the Okazaki fragments in the lagging strand, and also the nick between the DNA-replaced primer and the main strand.
- Prokaryotic polymerases:
- Pol I: removes RNA primer and replace it with DNA.
- Pol II: repair functions, SOS repair.
- Pol III: the main enzyme for polymerization because it works really fast and have proof-reading capability.
- Pol IV, V: repair functions, SOS repair.
- Eukaryotic polymerases:
- α: primase, but also adds a few DNA to the newly-made primer.
- β: repair.
- γ: mitochondrial polymerase.
- δ: lagging strand. Protein PCNA attaches to it with the help of RFC. Proofreads.
- ε: leading strand. Proofreads.
- Unwind supercoils
- Unwinding of double helix introduces supercoiling ahead of the replication fork.
- Topoisomerase unwinds supercoils by cutting DNA.
- Class I topoisomerase cuts 1 strand, class II cuts both strands of double helix.
- DNA Gyrase is the prokaryotic class II topoisomerase.
- Mutation
- Can be caused by spontaneously, random polymerase errors, by chemicals, radiation, insertion sequence and transposons. Things that cause mutation are called mutagens.
- Spontaneous mutations and polymerase errors:
- Purines undergo depurination, where the purine base comes off. Purines are large, so they fall off!
- Deamination: cytosine → uracil. 5-methylcytosine → thymine. Adenine → hypothanthine (analogous to G). Guanine → xanthine (analogous to G). Causes polymerase to incorporate wrong nucleotide.
- Non-watson-crick base-pairing due to tautomeric structures of bases: G-T and A-C. Causes polymerase errors by incorporating the wrong nucleotide.
- Long stretchs of polyN (eg. AAAAA) causes slipping of base-pairing, resulting in insertions or deletions.
- Chemicals:
- 5-bromouracil (5BU): can pair with G.
- Nitrous acid (HNO2): deaminating agent.
- Hydroxylamine (NH2OH): hydroxylating agent. Cytosine → hydroxylaminocytosine (analogous to T).
- Methylmethane sulfonate (MMS): alkylating agent. Guanine → O6-methylguanine (analogous to A).
- Intercalating agents (proflavin, acridine, ethidium bromide): causes insertions (if intercalate in template) or deletions (if intercalate in new strand). Results in frameshift.
- UV causes thymine-dimers.
- Insertion sequence and Transposons (nonhomologous recombination)
- If insert into gene, it nullifies the gene.
- If insert into promoter, it can either increase or decrease transcription of the gene.
- Somatic mutation = in somatic cells, not passed on to next generation.
- Germ-line mutation = in germ-line cells, passed on to next generation.
- Point mutations = at the level of a single or a few bases.
- Substitution: substituting one base for another.
- Transition: purine → purine, or pyrimidine → pyrimidine.
- Transversion: purine → pyrimidine, or pyrimidine → purine.
- Missense: results in changing one amino acid to another.
- Nonsense: results in changing an amino acid into a stop codon.
- Silent: causes no change in coded amino acid due to degenerate nature of codons.
- Neutral: causes change in coded amino acid, but does not affect the resulting protein's function. This is because of similarity of some amino acids. For example, lysine and arginine are both basic amino acids.
- Addition/Insertion: inserts a base.
- Deletion: deletes a base.
- Frame shift: caused by insertions and deletions, causes an entire shift in reading frame of all subsequent codons.
- Forward mutation (eg. A→G) can be reversed by a reverse mutation (G→A).
- Suppressor mutation: a type of reverse mutation that occurs at a different site from the original mutation.
- Chromosomal mutations = at the level of the chromosome.
- Insertion or deletion of whole DNA segments.
- Duplication of whole DNA segments.
- Inversion: segment of DNA breakes off, then reattach in the opposite orientation.
- Translocation: segment of DNA breaks off, then reattach else where.
- Repair
- Proof-reading: during DNA synthesis, the polymerase recognizes if a mismatch occurs, removes wrong nucleotide (3'-5' exonuclease activity) and replace it with the correct one.
- Transcription-coupled repair: during transcription, when RNA Pol II encounters DNA damage, will recruit repair enzymes.
- Mismatch repair:
- Recognize mismatch
- Remove, correctly, a stretch of DNA containing the mismatch.
- In prokaryotes, the newly made, unmethylated strand is recognized (by MutL,S,H) and removed (exonuclease).
- In eukaryotes, mechanism largely unknown.
- In humans, mismatch repair genes (aka mutator genes), are involved. Mutation of even one mutator gene (autosomal dominant) causes Hereditary NonPolyposis Colon Cancer (HNPCC).
- Remake the removed strand: polymerase, ligase.
- Nucleotide-excision repair: fixes UV damage like thymidine dimers. If both gene copies faulty (autosomal recessive), causes xeroderma pigmentosum (skin exposed to UV damage).
- Remove chunk of DNA by ABC excinuclease.
- Patch it up with polyerase and ligase.
- Base-excision repair:
- Remove damaged base by glycosylase, leaving AP (apyrimidinic) site.
- Remove backbone of damaged base by AP endonuclease.
- Remove a few more nucleotides by excision exonuclease.
- Patch it up by polymerase and ligase.
- SOS response in E. coli (aka translesion DNA synthesis): template is too damaged during replication, so pol II synthesizes DNA over these damages by randomly inserting nucleotides.
- Methylguanine methyltransferase in E. Coli removes alkylating mutation.
- Photolyases (not present in humans) reverse thymine dimers in the presence of light.
- Degradation
- Inactivation
- Binding to histones inactivates DNA because the promotor becomes inaccessible. To activate the DNA, histone acetylases or nucleosome remodeling complexes are needed.
- Binding of repressors, insulators.
- DNA methylation: cytosine → 5-methylcytosine.
- Gene silencing:
- RNA interference (gene knock out by siRNAs).
- Histone deacetylation (countered by histone tail methylation).
- DNA methylation of CpG islands in promoters.
- Imprinting.
- Prader-Willi syndrome: inactivation or deletion of paternal genes on chromosome 15. Symptoms: small + weak at birth, mental retardation, after 12 months develops uncontrollable hunger/eating, obesity, diabetes.
- Angelman syndrome: inactivation or deletion of maternal genes on chromosome 15. Symptoms: mental retardation, motor retardation, small head size, hyper activity, inappropriate laughter.
gene structure and organization; chromosomes; centromere, telomere
- Humans have 2N=46 chromosomes.
- Chromosomes: made of chromatin - DNA condensed by wrapping around histones. Looks like beads on a string.
- Histones: the majority of protein that binds chromosomal DNA.
- H2A, H2B, H3, H4 (2 of each) forms the histone core octamer.
- DNA + core octamer = the nucleosome (the bead).
- H1 binds to space regions (the string).
- Histone is positively charged (basic amino acids), which binds negative DNA.
- Centromere: region on chromosome where mitotic spindle attaches.
- Telomere: the ends of the chromosome.
- Gets shorter after each round of replication because the 5'-most RNA primer can't be replaced with DNA.
- In germ-line cells, telomerase lengthens telomere, but not in somatic cells.
- Consists of heterochromatin made up of tendemly repeated DNA sequences.
recombination, insertion sequences, transposons
- Recombination
- Meiosis I: synapsis occurs, bringing homologous chromosomes together. Results in formation of synaptonemal complex, which is a bivalent (tetrad) because of the 2 homologous chromosomes (with 4 chromatids). Crossing over occurs at the chiasma.
- Holliday model:
- Cut a single strand in each chromosome arm.
- Rejoin, but switch arms.
- Cut and rejoin again. If this cut is made on the same strand that was previously just cut, then no recombination results. But if cut is made on the previously uncut strands, the recombination results.
- Insertion sequences (aka IS elements):
- Simplest transposable elements. Found in prokaryotes.
- Contains transposase gene flanked by inverted repeats.
- Inserts into target site by making a staggered cut (like an endonuclease), and then insert.
- Polymerase uses staggered ends as templates. Thus, target site is duplicated.
- Can move from plasmid to genome and vice versa.
- Transposons: more complex than IS elements. Found in both prokaryotes and eukaryotes. Contains other genes in addition to transposase (or resolvase), flanked by inverted repeats.
mechanisms of genetic exchange, including transformation, transduction, conjugation, crossover,
recombination, linkage
- Transformation: cells scavenging DNA.
- Mechanism of transformation:
- Cell take up DNA.
- DNA pairs up with its homologous counterpart on the chromosome.
- Crossover occurs.
- Competent cells = cells able to take up DNA by transformation.
- Natural: some cells like B. subtilis is naturally competent.
- Treatment with chemicals.
- Electroporation.
- Transduction: virus transfers DNA from one cell to another.
- Virus infects cell: host DNA degraded into fragments, viral DNA takes over control.
- Host DNA fragment gets packed into virus progeny by accident.
- Virus progeny infects another cell, injects previous host's DNA fragment.
- Fragment enters cell, find its homologous counterpart, and crossover.
- Conjugation: transfer of genetic material via pillus.
- F+ (F factor as a plasmid) and Hfr strains (F factor integrated into chromosome) are capable of making pillus.
- Transfer of genetic material is one way, from the F+ (or Hfr) to the F-.
- F+ transfer an F factor to F- cell.
- F' = F+ + gene. Forms when integrated F factor comes out of the chromosome while taking a piece of the chromosome with it. This gene can be transferred to F- cell.
- Hfr strain transfers part of the F factor plus some chromosomal genes with it. This is because transfer of the integrated F factor requires the transfer of the entire chromosome, which never reaches completion. Genes from the donor pairs up with their homologous counterparts in the recipient and undergo crossover.
- Crossover:
- Holliday model = mechanism of crossover
- Pair up homologous DNA.
- Single-strand cut on each homologous DNA, then exchange and reseal.
- Repeat cut-exchange-reseal, but on the previously uncut strands in order for recombination to occur. If this is done on the previously cut strands, no recombination results.
- Single crossovers result in 2/4 recombinants (involves two adjacent chromatids in the tetrad).
- Double crossovers:
- No recombination if the same two strands are involved (the second crossover reverses the first).
- 2/4 recombinants if 3 chromatids are involved.
- 4/4 recombinants if all 4 chromatids are involved.
- Recombination:
- Homologous recombination:
- Crossover during meiosis I.
- Crossover in transformation, transduction, and conjugation.
- Nonhomologous recombination:
- Integration of IS elements and transposons.
- Linkage:
- Independent assortment randomnizes (unlinks) genes on different chromosomes.
- Homologous recombination unlinks, to some extent, genes on the same chromosomes (unlinks to a greater extent genes further apart from each other).
- This leaves genes on the same chromosome, especially those close together, with linkage.
- Genes closer together on the same chromosome → More linkage → more parental genotypes / less recombinant genotypes.
plasmids and bacteriophages
- Plasmids
- Circular, double-stranded, extrachromosomal DNA elements.
- Independent replication.
- Used as cloning vector, engineered to contain:
- Origin of replication.
- Selectable marker (usually antibiotic resistance).
- Restriction site (to insert your genes in).
- Bacteriophages = viruses that infect bacteria.
- Virulent: follows lytic life cycle.
- Temperate: can follow either lytic or lysogenic life cycle.
- Responsible for transduction in bacteria.
|
|